find the number of electrons co|19.10: Counting Electrons : Tagatay Because the sum of the numbers of protons and neutrons equals the mass number, 127, the number of neutrons is 74 (127 − 53 = 74). Since the iodine is added . List of towns and cities in England by historical population, the development of urban centres in England and before England through time. Settlements in ceremonial counties of England by population, places with 5,000 or more residents by county and the highest populated built-up area in each county.
PH0 · Protons, Neutrons, Electrons for Cobalt (Co, Co2+, Co3+)
PH1 · How to find the Number of Protons, Electrons, Neutrons for Co
PH2 · How to Find Number of Protons, Neutrons, and Electrons
PH3 · How to Find Electrons: 6 Steps (with Pictures)
PH4 · How To Calculate The Number of Protons, Neutrons, and Electrons
PH5 · How To Calculate The Number of Protons, Neutrons,
PH6 · Find the Number of Electrons Co
PH7 · Electron counting
PH8 · CO Lewis structure, Hybridization, and Molecular
PH9 · 2.6: Protons, Neutrons, and Electrons in Atoms
PH10 · 19.10: Counting Electrons
The Raxabago Police Station of the Manila Police District reported Wednesday that 20-year-old Paul Cedrick Diwa, one of the suspects in the alleged rape, invited the girl to a drinking session in .Check your IELTS results here. Find out how and when you will receive your TRF for the IELTS on paper and IELTS on computer, and the IELTS Life Skills test.
find the number of electrons co*******How to find the Number of Protons, Electrons, Neutrons for Co (Cobalt) - YouTube. Wayne Breslyn. 768K subscribers. Subscribed. 77. 9K views 3 years ago. In this video .
To find the number of electrons an atom has, start by looking up the element you're working with on the periodic table and .To find the number of electrons in cobalt cobalt, first locate the element on the periodic table. Next, find the atomic number which is located above the element 's symbol. Since .
Because the sum of the numbers of protons and neutrons equals the mass number, 127, the number of neutrons is 74 (127 − 53 = 74). Since the iodine is added .Recall that the charge on 1 mol of electrons is 1 faraday (1 F), which is equal to 96,486 C. We can therefore calculate the number of moles of electrons transferred when a known .
In chemistry, electron counting is a formalism for assigning a number of valence electrons to individual atoms in a molecule. It is used for classifying compounds and . To draw the Lewis structure of CO, it is vital to know the total number of valence electrons of the molecule. The molecule has one Carbon atom and one oxygen atom. We will first find out the valence .The easiest way to find the atomic number, is to look on a periodic table, the atomic number is in the upper left corner, or is the largest number on the square. Finding the .The Number of Protons from Electrons. For a neutral atom, the number of protons and the number of electrons are equal. This is what makes the atom charge-free. Therefore, you can determine the number of protons if .
CO Valence Electrons. To draw the Lewis structure of CO, it is vital to know the total number of valence electrons of the molecule. The molecule has one Carbon atom and one oxygen atom. We will first .Find the number of unpaired electrons in the t2g set of d -orbitals of [M n(H 2O)6]2+. View Solution.Co 2 + (d 7) in [I I C O (SCN) 4] 2 − has outer electronic configuration 3 d 7 or e 4 g t 3 2 g. e g orbitals have lobes present along the axis. They contain 0 unpaired electrons. Thus, the number of unpaired electron present in the d-orbitals (whose lobes are present along the axis) for the complex [CO(SCN) 4] 2 − is 0.In both the complexes, [Co(NH 3) 6] 3 + and [CoF 6] 3 −, the central Co atom is in +3 oxidation state. Ammonia is a strong field ligand. In [Co(NH 3) 6] 3 +, ammonia cause spin pairing. Cobalt has 3 d 6 outer electronic configuration which can also be written as t 6 2 g e 0 g. Thus, all the electrons are paired.And electrons in an atom is equal to its atomic number. In CO2, there are one carbon and two oxygen atoms and the number of electrons in them are: In C - 6 electrons. In two O's - 2×8 electrons. Total in CO2−6+(2×8)= 22 electrons. Suggest Corrections.
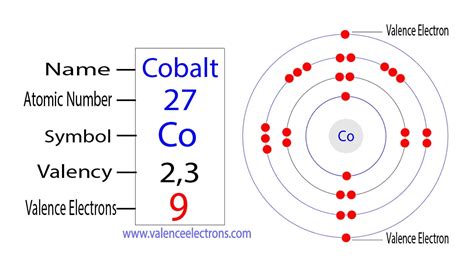
To find the number of valence electrons for Cobalt (Co) we need to look at its electron configuration. This is necessary because Co is a transition metal (d.
find the number of electrons co 19.10: Counting Electrons To find the number of valence electrons for Cobalt (Co) we need to look at its electron configuration. This is necessary because Co is a transition metal (d. In the case of first row transition metals, the electron configuration would simply be [Ar] 4s x 3d x. The energy level, "n", can be determined based on the periodic table, simply by looking at the row number in which the element is in. However, there is an exception for the d-block and f-block, in which the energy level, "n" for the d block is . 2. Find the electron configuration for the element you are examining. Once you know an element's electron configuration, finding its number of valence electrons is quite simple (except, of course, for the transition metals.) If you're given the configuration from the get-go, you can skip to the next step.
find the number of electrons coThe rule is to count all of iron's valence electrons as d-electrons. Iron is in group 8, so. group 8 - 3+ charge = d 5 (or 3d 5) 8 - 3 = 5. Structure of the octahedral ferricyanide anion. Because the overall charge of the complex is 3-, Fe is in the +3 oxidation state and its electron count is 3d 5. The same procedure can be applied to any . In this video we’ll use the Periodic table and a few simple rules to find the protons, electrons, and neutrons for the element Cobalt (Co). From the Periodic.
Rules to Finding Number of Protons, Neutrons, and Electrons # of protons = atomic number # of neutrons = mass number – atomic number # of electrons = atomic number – charge. That’s it! Examples. Great, lets apply the rules to some examples. # of protons = 17 # of neutrons = 37 – 17 = 20 # of electrons = 17 – 0 = 17First, to find the number of protons, we need to realize that the neutral atom had 53 electrons because it is the additional one electron that makes it a 1- anion. Now, because the atom has 53 electrons, it must also . To determine the number of lone pairs (unbonded pairs) and bonding pairs of electrons for CO we first need to draw as valid Lewis Structure. Once we have a L.19.10: Counting Electrons The magnetic moment is related to the number of unpaired electrons according to the following relation: Magnetic moment, μ = √ n ( n + 2 ) B M where, n = number of unpaired electrons. B M stands for Bohr magneton, a unit of the magnetic moment.Find the number of unpaired electron (s) in the complex ion [ C o F 6] 3 −. View Solution. Click here:point_up_2:to get an answer to your question :writing_hand:49 find the total number of unpaired electrons int2g orbitals of central atom in cof.
Solution: Total number of valence electron in N a2C O3. = 2× 8+24 = 40. N a+ = 8,C O32− = 24. Number of valence electron in 106g of N a2C O3. = 40 ×6.023 ×1023. Number of valence electron in 0.53g of N a2C O3. = 0.2× 6.023× 1023 = 1.2046×1023. Find the number of valence electrons present in 0.53 gram of Na 2 CO 3. And you have one more electron to worry about. And so that electron would go into a 3S orbital. So the full electron configuration is 1S2, 2S2, 2P6, and 3S1. When I want to figure out .
With over 35 clinics operating in Singapore, Malaysia, Indonesia, China, UK and Europe, we are the trusted leader in chiropractic care. BOOK AN APPOINTMENT. About Us. Our Story; Our Clinics; Our Doctors; Join Our Team; . Dr Shane who is a caring and professional chiropractor. I have been with CFG since Feb 2013 and under Dr Shane .
find the number of electrons co|19.10: Counting Electrons